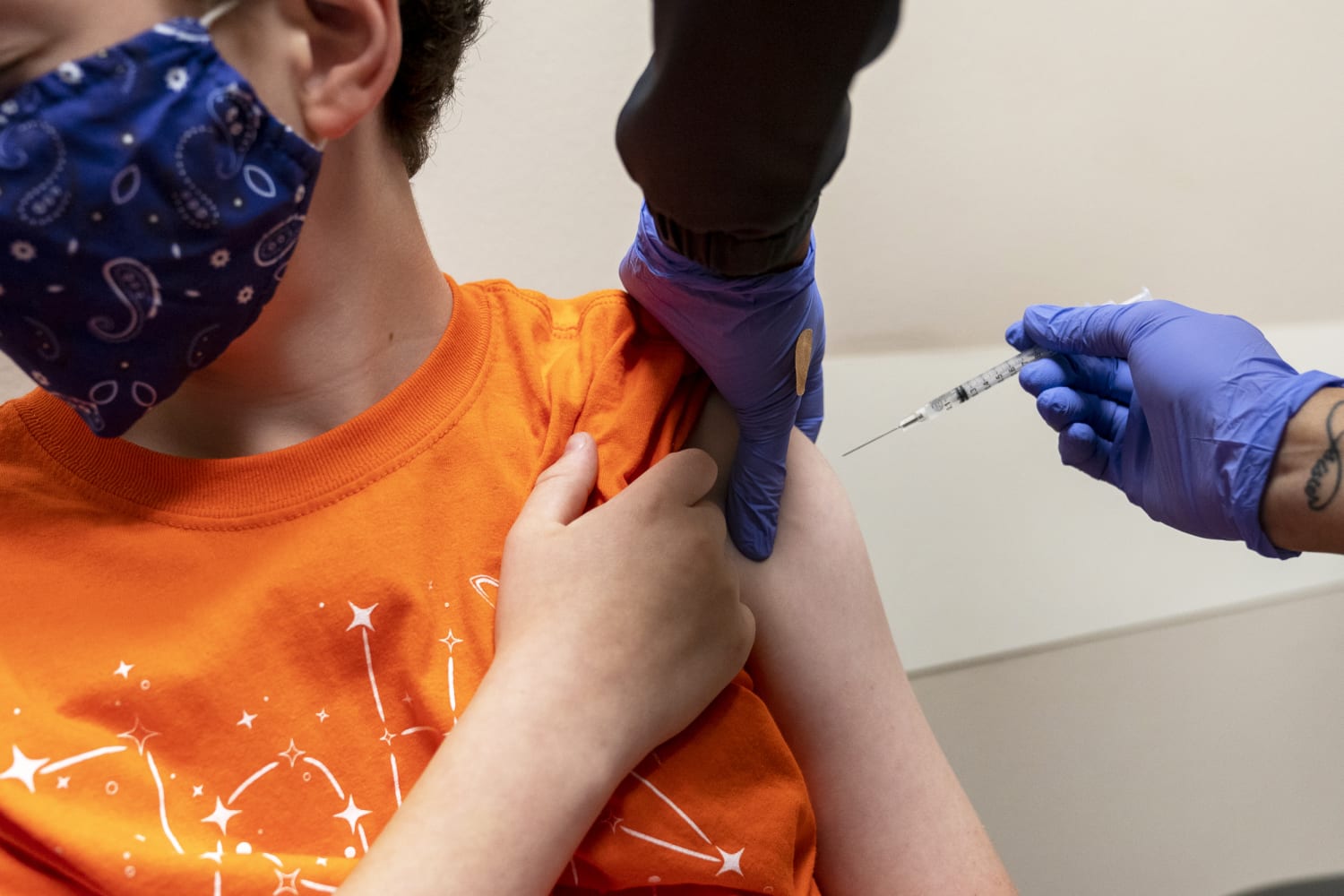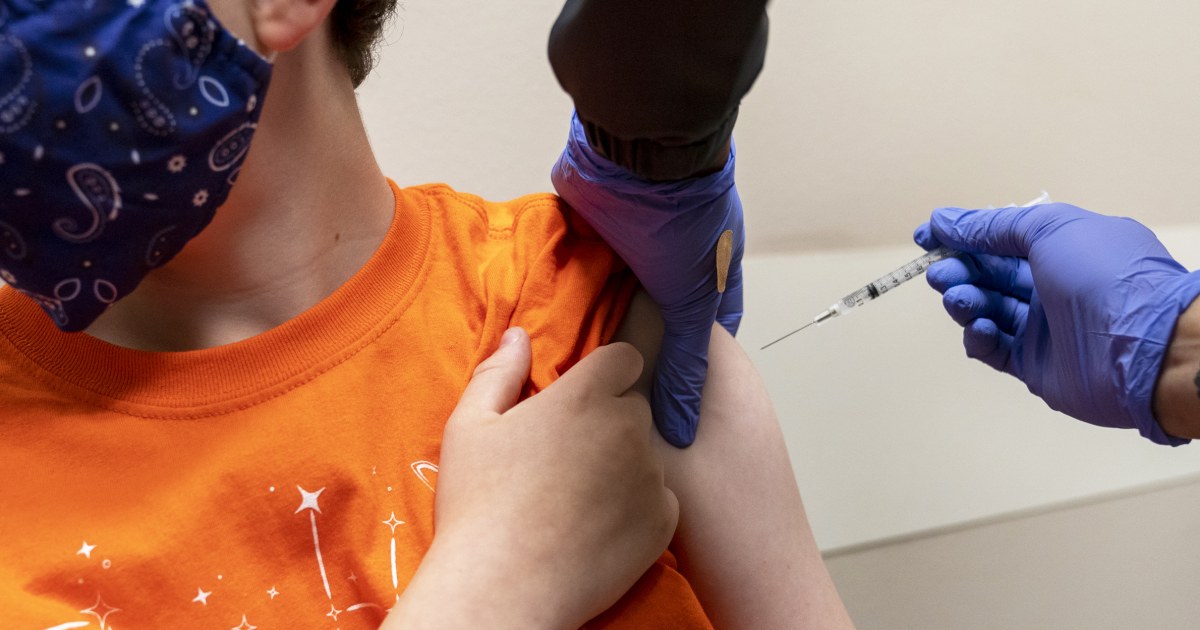
The Food and Drug Administration on Friday authorized both Pfizer-BioNTech and Moderna’s Covid-19 vaccines for young children, though the Centers for Disease Control and Prevention still needs to sign off before shots can be administered.
The Pfizer vaccine was authorized for children ages 6 months to 4 years, while the Moderna vaccine was authorized for children ages 6 months to 5 years, the FDA said in a statement. It also authorized Moderna’s shot for kids ages 6 to 17.
Full coverage of the Covid-19 pandemic
The agency’s authorization brings the roughly 18 million children under age 5 in the United States one step closer to getting vaccinated. Children in the age group are the only remaining group in the country ineligible to get the shots.
The FDA’s decision will now go to the Centers for Disease Control and Prevention’s advisory committee, which will meet Friday and vote Saturday to decide how the vaccines should be used for children under 5. If all goes as planned, CDC Director Dr. Rochelle Walensky will sign off on the authorization, potentially within hours of the committee’s clearance. Shots in arms could begin as early as next week.
On Wednesday, the FDA’s vaccine advisory committee unanimously recommended that the agency authorize both the Pfizer and Moderna vaccines for the nation’s youngest children.
While many members of the committee noted that severe illness from Covid is less common in children than it is in adults, they said the shots should still be made available as an option for parents who wish to vaccinate their children.
Pfizer’s vaccine regimen for little kids is two doses, given three weeks apart, followed by a third dose at least two months later. The shots are 3 micrograms each, one-tenth the dosage given to adults. Clinical trials found the shots to be 80% effective in preventing symptomatic Covid.
Moderna uses two 25 microgram doses, a quarter of the dosage given to adults. The shots are given four weeks apart. The shots were shown in clinical trials to be 40 to 50% effective at preventing milder infections, though the company has said it is testing a booster dose for the age group that could be distributed sometime this fall.
The most common side effects reported from the shots were pain at the injection site, irritability and drowsiness.
Follow NBC HEALTH on Twitter & Facebook.
Source: | This article originally belongs to Nbcnews.com