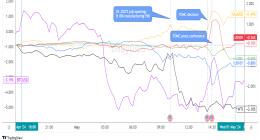
World Health Organization officials expressed confidence that AstraZeneca PLC’s Covid-19 vaccine can prevent severe cases of the disease, as well as hospitalizations and deaths, despite questions about the protection it offers against a fast-spreading strain of the virus first detected in South Africa.
The remarks followed a release of information over the weekend about a small clinical trial of the vaccine in South Africa, which prompted the government there to halt a planned rollout of the shot.
The preliminary data, which hasn’t been published in detail, suggested the vaccine may not prevent mild and moderate cases of Covid-19 from a new variant that has become the dominant version of the virus in South Africa and the broader southern African region.
The WHO’s director-general, Tedros Ghebreyesus, said the trial’s findings were “clearly concerning news,” but stressed that they came with “important caveats.”
Those included the small number of volunteers in the trial—around 2,000—and their relatively young median age of just 31 years. WHO officials and outside researchers said the trial was too small and its volunteers too young to draw clear conclusions about the vaccine’s efficacy against the variant, known as B.1.351, especially when it comes to preventing more severe cases of Covid-19. Younger people tend to suffer from milder versions of Covid-19 and experience severe cases less often.