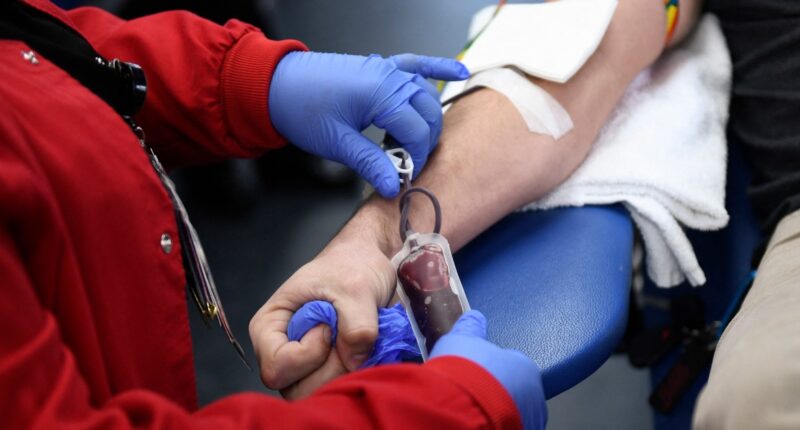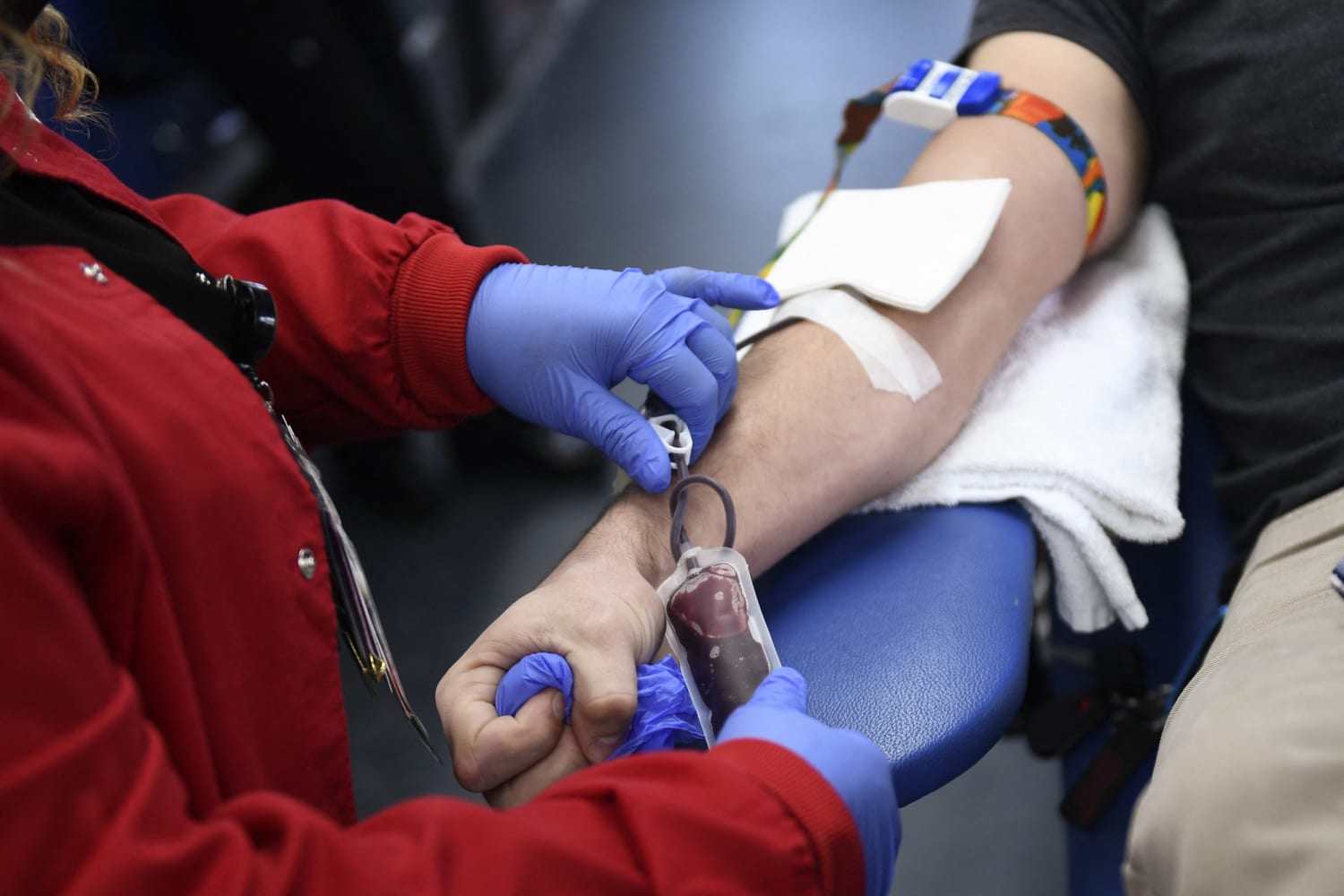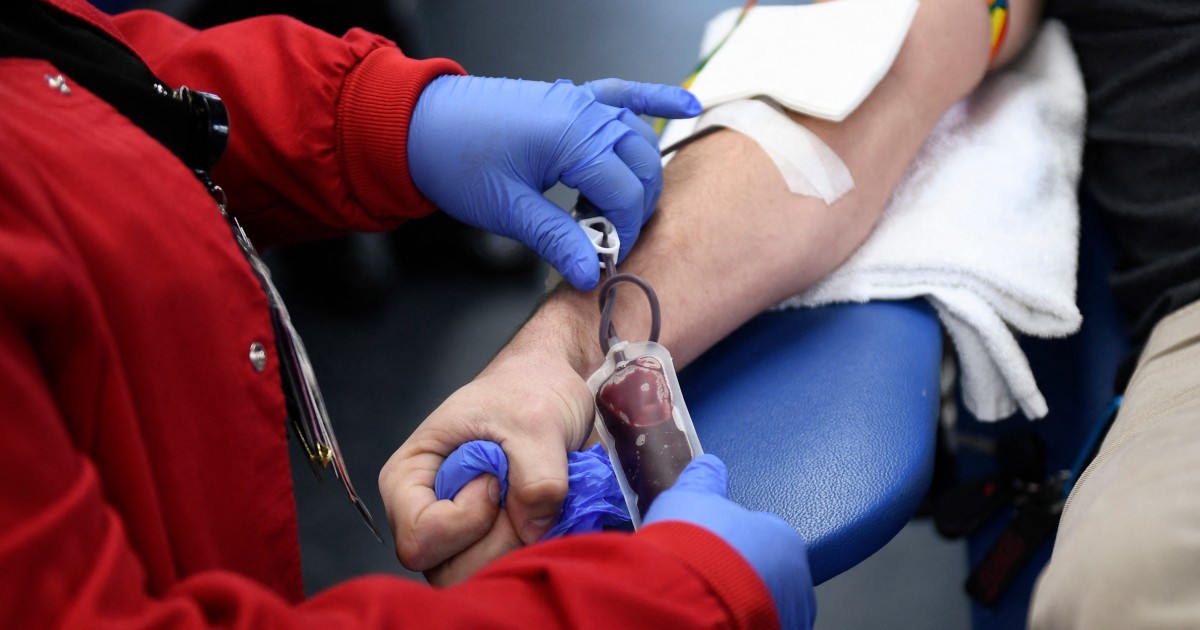
The Food and Drug Administration said Thursday that it finalized a new rule that will allow more gay and bisexual men to donate blood.
Under the latest guidelines, all potential donors would need to complete an individualized risk assessment — regardless of gender or sexual orientation. People who have had anal sex with a new partner, or more than one partner, in the last three months would be asked to wait to donate blood.
The updated guidelines mean most gay and bisexual men who are in a monogamous relationship with another man will no longer need to abstain from sex to donate blood.
Previously, the FDA only allowed donations from men who have sex with men if they hadn’t had sex with another man for three months.
“The implementation of these recommendations will represent a significant milestone for the agency and the LGBTQI+ community,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a release.
The agency will continue to monitor the safety of the blood supply, he added.
The FDA’s restrictions on blood donations from men who have sex with men stem from the AIDS crisis, which began in the early 1980s, when little was known about HIV.
The agency first proposed the new rules, which are in line with those in Canada and the United Kingdom, in January.
People who are taking medication to prevent or treat an HIV infection would be asked to wait to donate blood under the new guidelines.
Follow NBC HEALTH on Twitter & Facebook.
Source: | This article originally belongs to Nbcnews.com