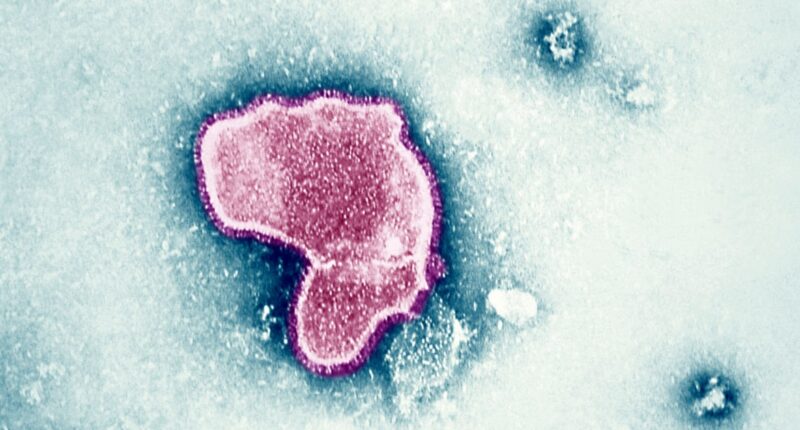
The Food and Drug Administration on Wednesday approved the world’s first RSV vaccine: a shot for adults ages 60 and up, made by pharmaceutical giant GSK.
The milestone was decades in the making. Researchers first attempted to develop a vaccine for respiratory syncytial virus roughly 60 years ago.
In a late-stage clinical trial, the single-dose shot lowered the risk of symptomatic illness by 83% and of severe illness by 94%.
The Centers for Disease Control and Prevention must still recommend the vaccine before it becomes available to the public. A CDC advisory committee is scheduled to meet in June to discuss how the shot should be used.
Dr. Phil Dormitzer, GSK’s senior vice president and global head of vaccines research and development, said the company is already manufacturing doses.
“The goal is to have shots available in the fall so that people can get them before the next RSV season coming up late fall and winter,” Dormitzer said, though he did not give specific production numbers.
RSV causes a lower respiratory illness that is typically mild for healthy adults. But in serious cases, the virus can lead to pneumonia or bronchiolitis, which inflames airways and clogs them with mucus. Older people and infants are particularly at risk: RSV kills up to 10,000 people ages 65 or older and up to 300 children under 5 every year in the U.S.
RSV cases typically peak between late December and mid-February, but cases last year started climbing in the summer as people left pandemic precautions behind.
As of late April, the rate of RSV hospitalizations for the current season was around 51 per 100,000 people, according to the CDC. That’s up from 19 hospitalizations per 100,000 people by the same point in the prior season.
GSK’s trial showed that protection from its vaccine lasted at least six months.
“It’s not like you have to wait to the very last minute because it’s not going to last long enough,” Dormitzer said. “We know from studies we have done that getting a vaccine — for example, in the fall — should cover you through the next RSV season.”
Dormitzer said the company’s data also suggests it is safe to administer the RSV vaccine at the same time as a standard flu shot. But GSK is still studying whether the vaccine can be administered alongside high-dose or adjuvanted flu vaccines (shots with an added ingredient to encourage a better immune response), which are the types the CDC recommends for older adults.
Monitoring for safety concerns
GSK’s trial is ongoing, so the company plans to keep collecting data over the next two RSV seasons. It will also monitor people who receive the newly approved shot.
So far, commonly reported side effects in the trial include injection site pain, fatigue and muscle pain.
The FDA noted a higher incidence of atrial fibrillation (irregular heart rhythm) among vaccine recipients relative to the control group, and it identified one case of Guillain-Barré syndrome — a rare neurological disorder that damages nerve cells and causes muscle weakness or paralysis — that was potentially related to the vaccine.
Dormitzer said it’s hard to know what to make of the Guillain-Barré case but added, “I would not say it’s a great concern at this point.”
A panel of FDA advisers in March voted 10-2 in favor of approving the vaccine based on its safety and unanimously recommended the vaccine based on its efficacy.
The future of RSV vaccines
A second RSV vaccine for older adults, from Pfizer, is up for FDA approval later this month. The FDA advisory panel voted 7-4 to recommend that shot based on its safety and efficacy.
Some members expressed concerns that not enough participants in Pfizer’s trial got infected with RSV to adequately assess the shot’s efficacy, and several worried about a potential association with Guillain-Barré. One man in Pfizer’s trial developed Guillain-Barré after he received the vaccine, and a woman developed Miller Fisher syndrome, a related, rare nerve disease.
The FDA has asked Pfizer to conduct a study on the risk of Guillain-Barré after its vaccine is approved.
Pfizer also tested a second application of its shot in pregnant people to help protect infants from RSV. The FDA is reviewing that data, with a decision likely in August.
Dormitzer said GSK doesn’t have further plans to study its shot among pregnant people after a previous trial involving a slightly different version of the vaccine showed a higher preterm birth rate among some vaccine recipients.
However, GSK is studying its shot in people ages 50 to 59 — particularly those with underlying health issues. Dormitzer said that data should be available sometime after July.
Source: | This article originally belongs to Nbcnews.com