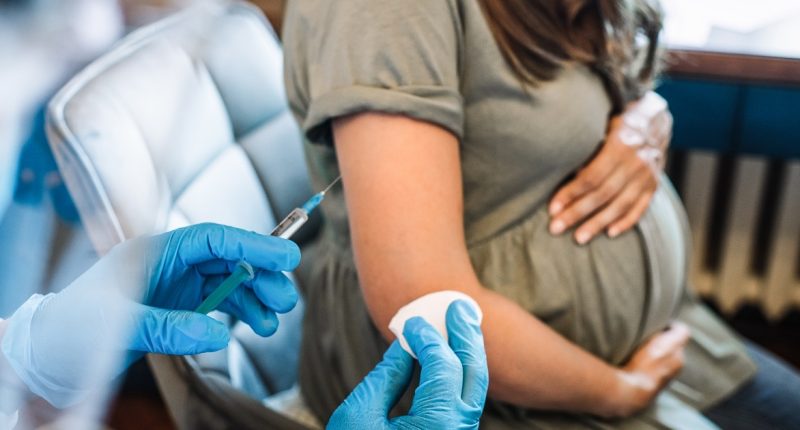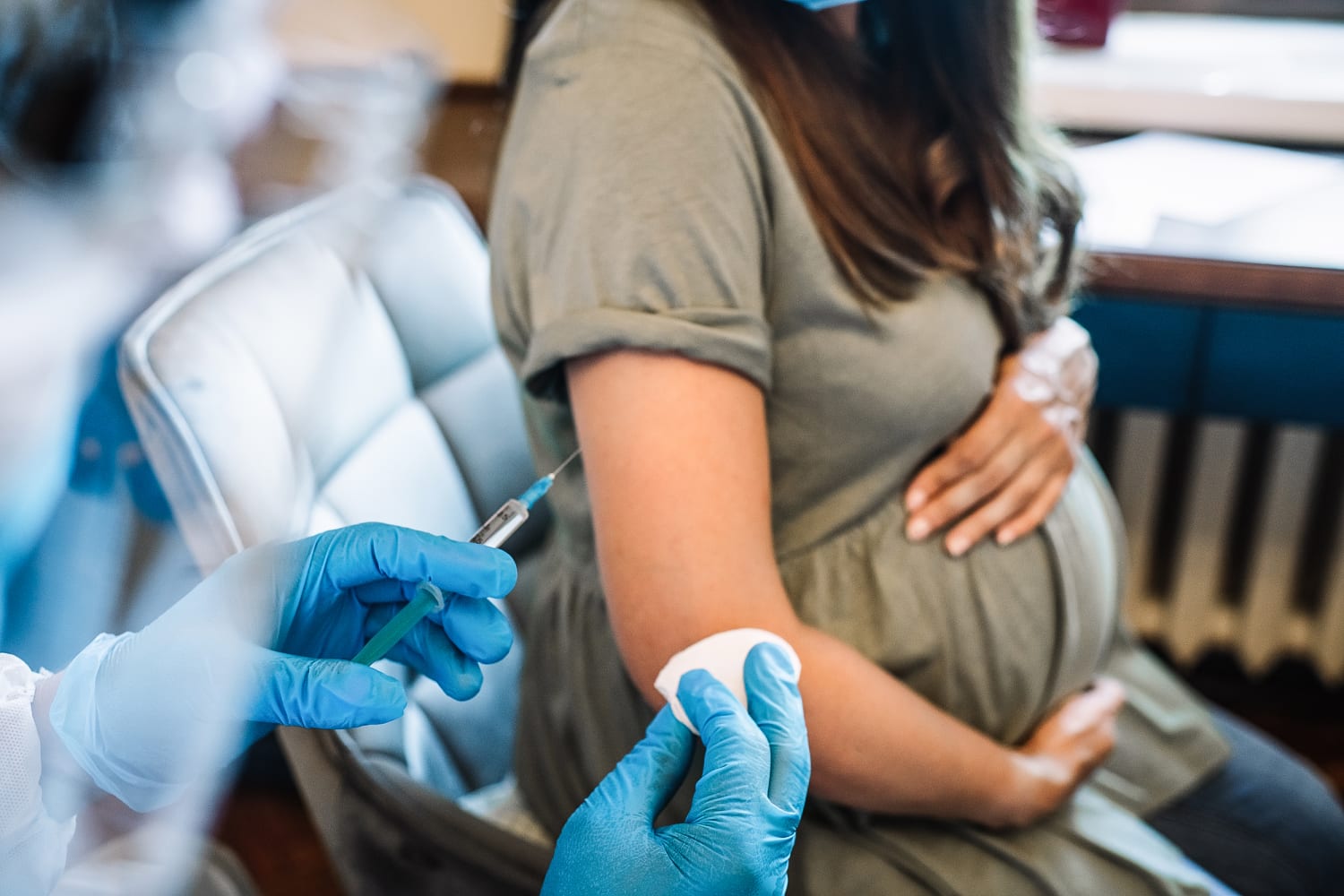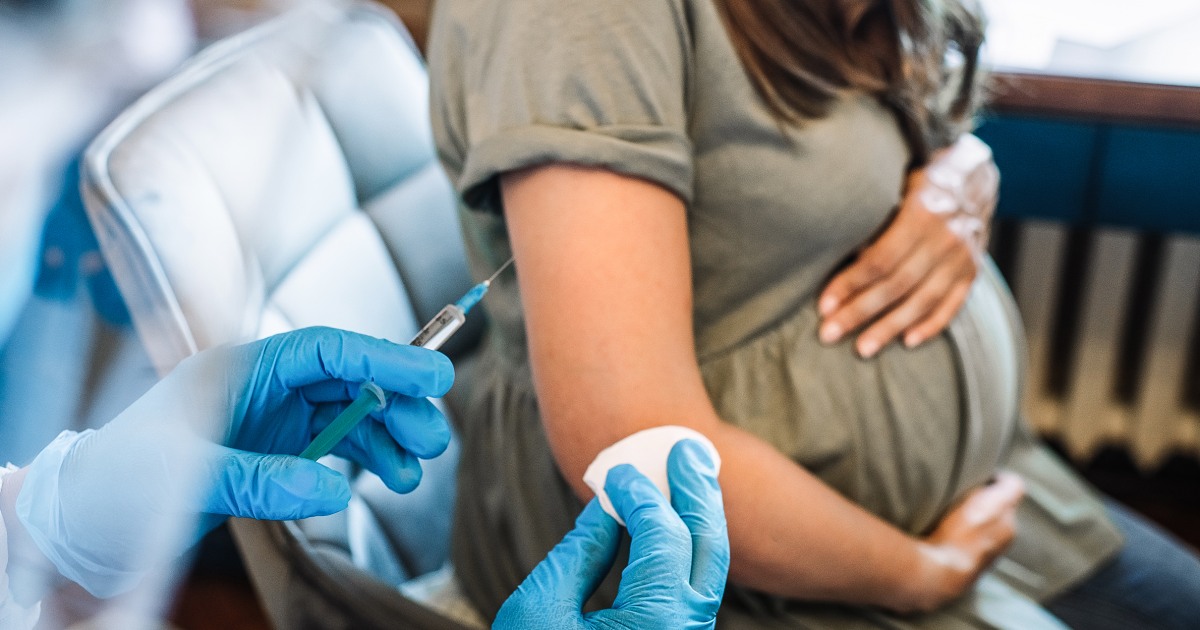
The Food and Drug Administration on Monday approved a vaccine that is administered to pregnant people to protect their babies from RSV, or respiratory syncytial virus, through their first six months.
The single-dose shot, made by Pfizer, spurs the production of protective antibodies that transfer from mother to infant through the placenta. It’s the first vaccine that can protect babies from RSV, which causes a lower respiratory illness that can be severe in infants.
However, last month the FDA also approved an injectable RSV drug for infants that delivers a dose of antibodies directly to the bloodstream.
Before Pfizer’s shot can be distributed to the public, an advisory committee to the Centers for Disease Control and Prevention must still recommend who should receive it. After that, it’s up to CDC Director Dr. Mandy Cohen to officially recommend the vaccine.
Pfizer said that the CDC advisory committee will most likely meet by early October and that the company plans to make the vaccine publicly available shortly after it is officially recommended.
The new shot, called Abrysvo, is approved for pregnant people at 32 to 36 weeks’ gestation. The same vaccine was approved in late May for people ages 60 and up and is already available at certain Walgreens locations.
RSV overall is mild, and most children have been infected by age 2. In serious cases, however, an infection can lead to pneumonia or bronchiolitis, which inflames airways and clogs them with mucus. Older adults and babies are particularly susceptible: RSV leads to up to 300 deaths a year among those under 5 in the U.S. and up to 160,000 hospitalizations among people 65 and up.
Last year’s RSV season brought a dramatic spike in severe illness that overwhelmed children’s hospitals. According to a study published this month, most babies who ended up in the intensive care unit with RSV in late 2022 were previously healthy and born full term.
Strong protection against severe disease — but for how long?
Tamara Zangrilli was among the first people to take Abrysvo while pregnant — she signed up for Pfizer’s clinical trial in 2021.
Zangrilli works as an operations coordinator at Children’s Hospital Colorado and was aware of the health risks of RSV among infants. Still, she said she thought carefully about whether to get an experimental vaccine.
“We really, really, really discussed it. My husband and I spent like three days just deciding on whether or not we wanted to do it,” Zangrilli said.
Ultimately, she said, her worry about her baby getting RSV won out.
In that clinical trial, which included nearly 7,400 participants, Abrysvo was found to lower the risk of severe disease from RSV among infants by 82% within roughly three months after birth. By around six months, the efficacy was around 69%.
“If brought into broad use, there will be children who otherwise would have been hospitalized, otherwise would have ended up on ventilators this winter, that won’t,” said Dr. Bill Gruber, senior vice president of clinical research and development for Pfizer.
The most common side effects reported among pregnant women who got the shot were fatigue, headache, injection site pain, muscle pain, nausea, joint pain and diarrhea.
In May, an FDA advisory committee reviewed data on the vaccine, and though the group generally praised its efficacy, there were some reservations about the duration of protection and a potential risk of pre-term birth. In the trial, there was a slightly higher rate of births before 37 weeks’ gestation among people who got the vaccine (5.7%) than among those who got a placebo (4.7%).
Although the difference wasn’t statistically significant, the prescribing label for the vaccine will come with a warning not to administer Abrysvo before 32 weeks’ gestation due to that numerical imbalance. Both of those observed rates were lower than the rate of preterm births in the general population: around 10%, according to the CDC. Pfizer has said it will continue to monitor the risk of preterm birth among vaccine recipients.
Trial participants weren’t told whether they received the vaccine or a placebo, but Zangrilli said she didn’t experience any side effects. She gave birth to her son, Harrison, when he was full term.
“Whether it’s pertussis, influenza, tetanus, there’s a long track record of safety for administering vaccines to pregnant women,” Gruber said.
Two new options for infants: A vaccine and an antibody drug
Unlike past RSV seasons, expectant parents and those with young babies now have two ways to protect their children.
The antibody injection, called Beyfortus, is CDC-recommended for babies up to 8 months old who are born during or entering their first RSV season, which typically starts around October. It’s also recommended for infants 8 to 19 months old who are at increased risk of severe disease and entering their second season.
Gruber said Abrysvo might produce a broader antibody response, however, since the process the vaccine spurs is closer to how the body would naturally defend itself against a virus. That may lower the chances that RSV escapes immunity from the vaccine relative to the antibody shot. But no data directly comparing the two exists yet.
Health authorities have not yet weighed in on whether pregnant mothers who plan to immunize their babies with Beyfortus should get the Abrysvo vaccine, but it may be a topic of discussion for the CDC’s advisory committee.
Zangrilli said Harrison is now 18 months old and has not tested positive for RSV thus far. She’s open to getting the vaccine again if health authorities suggest it for subsequent pregnancies, she said.
“The hope is in the next year to have another child and if this vaccine is available, and they’re like ‘we recommended it,’ I would absolutely do it,” she said.
Source: | This article originally belongs to Nbcnews.com