Fake Botox is behind 19 reports of vision problems and difficulty breathing and swallowing in at least nine states, the Food and Drug Administration said Tuesday.
The products in question were obtained from unlicensed sources, and “may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe,” the agency wrote on its website.
“The sources of these products are unverified and unknown, which is why this situation is certainly concerning,” said Michelle Waltenburg, senior botulism epidemiologist at the Centers for Disease Control and Prevention. Waltenburg is working with the FDA and state health departments in the investigation.
Cases have been detected in Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee and Washington.
There is no indication that authentic Botox, made by the drugmaker AbbVie, is linked to any of the illnesses. That product, the FDA said, “should be considered safe and effective for its intended and approved uses.”
Botox uses a purified form of a neurotoxin called botulinum toxin that paralyses muscles for a period of time. Injected into specific areas of the face, it can relax muscles that cause wrinkles.
The concern is that a counterfeit product may spread elsewhere in the body, potentially incapacitating muscles needed to breathe.
“Paralysis of respiratory muscles is a very serious consequence,” Waltenburg said. “That’s why we consider botulism to be both a medical and a public health emergency and encourage people who have symptoms of botulism to seek medical care immediately.”
At least 19 women developed symptoms after they got what they thought was real Botox, either from people who were never trained to give the shots or received them in “non-healthcare settings,” including homes or spas, the CDC said Monday.
Almost all sought out the injections for cosmetic reasons. Most were around age 40.
Nine of those patients had to be hospitalized. Four were given botulism antitoxin because doctors worried that the toxin could spread throughout the body. While some had difficulty breathing, none of the patients has to be put on a ventilator, Waltenburg said. No deaths have been reported.
The CDC is expected to alert physicians nationwide to the issue as early as Wednesday.
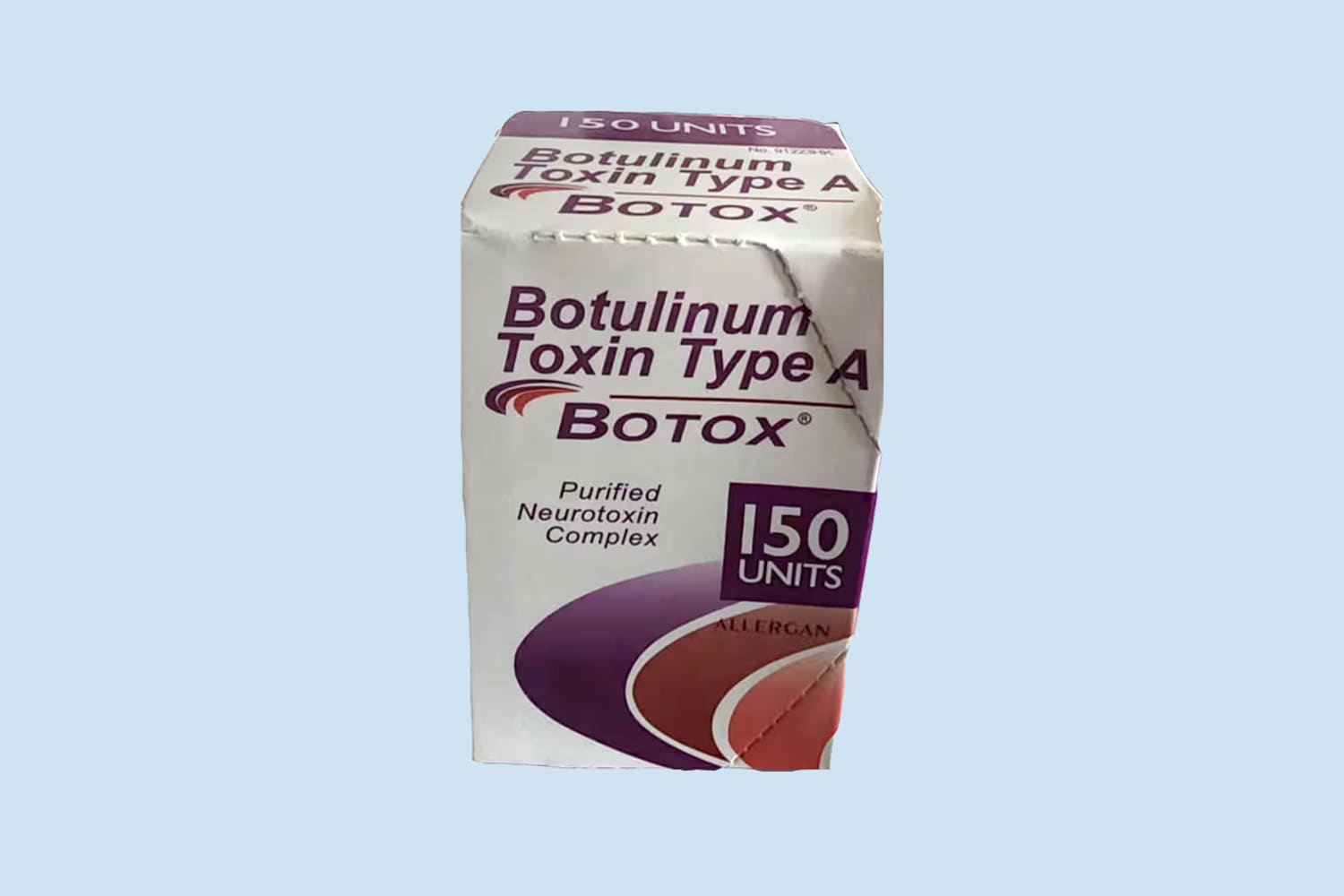
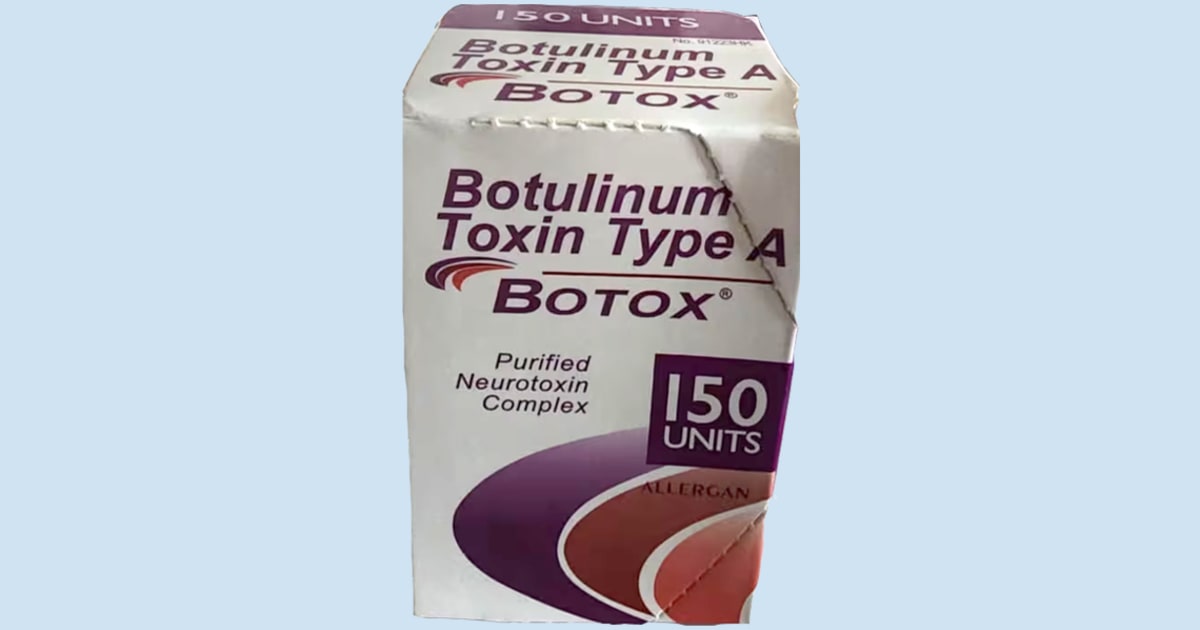
The FDA said there are several ways to identify counterfeit Botox products:
- The outer carton and vial contain lot number C3709C3.
- The outer carton displays the active ingredient as “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA.”
- The outer carton and vial indicate 150-unit doses, which is not a unit made by AbbVie.
- The outer carton contains language that is not English.
Source: | This article originally belongs to Nbcnews.com