John Wishman was diagnosed with the deadliest form of brain cancer, glioblastoma, in fall 2020.
Two and a half years later, he’s still traveling and enjoying life — a rarity for a cancer with an average survival time of just 12 to 18 months.
Wishman, 61, of Buffalo, New York, attributes that to an experimental vaccine that’s designed to delay the progression of the tumor. The vaccine, called SurVaxM, targets a protein found in tumors called survivin, named for the role it’s thought to play in the survival of cancer cells. Get rid of survivin, the thinking goes, and the cancer cells will die.
It sounds like a far-fetched dream: a vaccine that can delay the return of glioblastoma, one of the deadliest and treatment-resistant cancers. More than 14,000 people in the U.S. were diagnosed last year, according to Tom Halkin, a spokesperson for the National Brain Tumor Society, a nonprofit group. It accounts for almost half of all malignant brain tumors. The disease is devastating for patients and families; the five-year survival rate is 6.8%.

Wishman got the vaccine through an expanded access program — sometimes called compassionate use — that allows seriously ill patients to gain access to experimental medicines. His daughter Lydia is a nurse at Roswell Park Comprehensive Cancer Center, where researchers are studying the drug.
In an early clinical trial, SurVaxM was found to extend survival time for people diagnosed with the brain cancer to 26 months, on average. Now the drugmaker, New York-based MimiVax, is enrolling patients in a larger trial, hoping to confirm the results. The expanded access program is no longer available.
The new trial will enroll up to 270 patients. It is expected to take place at more than 10 sites in the U.S. and China and will compare the shot to patients who receive standard care.
Tracey Kassman, 65, enrolled in April 2022, three months after being diagnosed with glioblastoma. That same month, she received her first shot.
Kassman, a retired lawyer from Buffalo, now gets a shot once every two months. But because the trial is randomized and double-blinded, neither Kassman nor her doctors know if she’s getting the vaccine or a placebo.
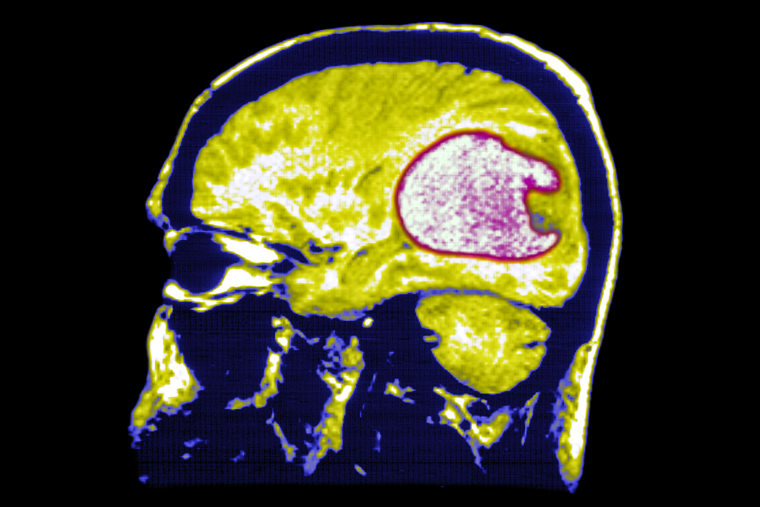
“It’s been at times a leap of faith,” she said, “because right before I get the shot, I have this MRI, and every time I have the MRI, I’m like, ‘OK, well this could be it.’”
Why is glioblastoma so hard to treat?
Glioblastomas are aggressive cancers: They grow quickly and tend to have invaded other parts of the brain and spinal cord by the time a person is diagnosed.
Surgical removal of the entire tumor is almost impossible.
“It’s like octopus tentacles reaching into other parts of the brain,” said Honggang Cui, an associate professor of chemical and biomolecular engineering at Johns Hopkins Whiting School of Engineering.
Treatment typically involves surgery, chemotherapy and radiation, Cui said. But unless every cancer cell is eliminated, the tumor often comes back in what’s referred to as recurrence.
SurVaxM works by training the immune system to target and attack the cancer cells, so if they do return, the body can pick them off, preventing a new tumor from growing, said Michael Ciesielski, the CEO of MimiVax.

The approach is “promising,” Cui said. “This could bring hope to people who are impacted by GBM.”
Participants in the trial will first have surgery to remove as much of the tumor as possible, followed by radiation and chemotherapy, with a drug called temozolomide, said Dr. Robert Fenstermaker, the chair of the neurosurgery department at the Roswell Park Comprehensive Cancer Center and co-creator of SurVaxM.
“There’s usually a hiatus of about a month while radiation is still working, and it’s during that phase that we like to start the vaccination because that’s when the immune system has been rejuvenated,” he said.
The vaccine — given in the arm just like a flu shot or Covid shot — consists of four doses, spread out over two months, followed by a booster dose every two months. Participants in the trial will either get the real vaccine for each shot or a shot of a placebo every time. Participants will also get a brain scan every two months to monitor for signs of progression.
A need for different approaches
SurVaxM isn’t the first attempt to create a vaccine to delay the recurrence of glioblastoma. Other cancer vaccines have targeted survivin, but none of them so far have reached mid- to late-stage clinical trials, according to Ciesielski.
Dr. Alyx Porter, a neuro-oncologist at the Mayo Clinic in Phoenix, said the approach is different from what’s been tried in the past.
Targeted therapies like checkpoint inhibitors, for example, have grown in popularity in recent years, improving survival in people with cancer including those with breast or lung cancers. But these drugs are far less effective for brain tumors, because they can’t cross the blood-brain barrier, a network of blood vessels that keeps foreign substances from entering the brain.
The belief, Porter said, is that the antibodies generated by a vaccine would be able to reach the brain. But, she added, “the proof will be in the pudding with the trial.”
Results are still a ways off: According to Ciesielski, the company doesn’t expect its earliest results from the Phase 2b trial until mid-2024, and the trial likely won’t be completed for another 18 to 24 months after that. If successful, the company will have to conduct a larger Phase 3 clinical trial.
The high mortality rates of glioblastoma “warrants people pressing the edge and seeking out new treatments and allowing us to really maximize where immunotherapy may benefit,” said Porter, who is not involved with the SurVaxM trial.
So far, the drug appears to be safe, Fenstermaker said. Known side effects from the vaccine include fever, itching, redness and muscle aches.
Ciesielski said the company is also looking to use the vaccine on other forms of cancer, including multiple myeloma and neuroendocrine tumors, a rare form of cancer that can develop wherever there are neuroendocrine cells, which are found in various organs including the lungs, pancreas and gastrointestinal tract.
For Kassman, of Buffalo, New York, she feels “incredibly lucky” for a chance at a possible treatment.
“I could have ignored this whole thing again for a couple of weeks,” she said, “and I might not be here to talk about this with you.”
Follow NBC HEALTH on Twitter & Facebook.
Source: | This article originally belongs to Nbcnews.com