The number of people with highly drug-resistant bacterial infections linked to contaminated eyedrops has reached 81, the Centers for Disease Control and Prevention reported Friday.
The 81 cases, up from 68 identified in March, include 14 people who have been blinded and four others who had to have their eyeballs surgically removed.
Though most infections have been limited to the eyes, the bacteria can be fatal when it enters the bloodstream. As of Monday, the CDC said, four people have died.
“These were catastrophic and life-altering infections,” Maroya Spalding Walters, who leads the CDC’s antimicrobial resistance team, said in an interview.
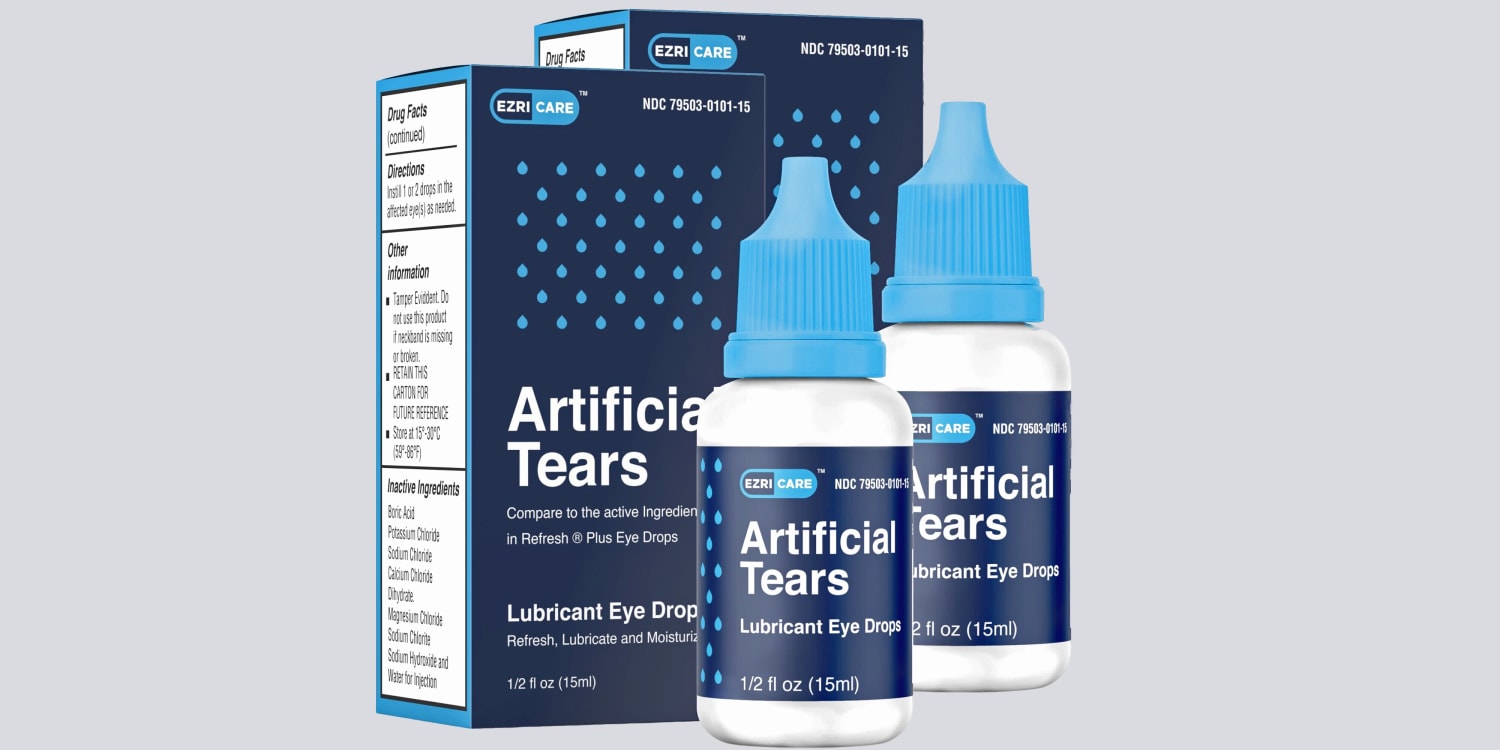
Though many patients said they’d used multiple brands of eyedrops, only EzriCare Artificial Tears, as well as Delsam Pharma’s Artificial Tears and Delsam Pharma’s Ointment, have been linked to the outbreak.
The products were manufactured by Global Pharma Healthcare in India, and sold mostly online.
The CDC expects the case count to rise, although the rate has slowed since the eyedrops were recalled by Global Pharma in February.
The infections come from a specific strain of Pseudomonas aeruginosa bacteria that has proven impossible to control with standard antibiotics.
Before last year, this particular form of the bacteria had never been reported in the United States.
Now, cases have been discovered in 18 states: California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, New Jersey, New Mexico, Nevada, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin.
The initial infections started popping up last year.
Cases were first noted in Connecticut in June. Doctors in Miami started seeing such infections late last summer. An Ohio woman became infected in November.
Some cases occurred among clusters of people living in long-term care facilities, the CDC said, even among patients who never used artificial tears.
Sometimes, the bacteria can entered a person’s body through the eyes via the eyedrops, but never impact the eyes.
Those bacteria can then set up shop in the body, colonizing in the respiratory or digestive systems for months without making the person sick.
That bacteria, however, can be transmitted to others through shared medical equipment, for example.
The Food and Drug Administration has also been leading an investigation into the contaminated drops. But the agency’s last update on the matter was Feb. 22.
The FDA did not respond to requests from NBC News for a more recent update.
Both the CDC and FDA have urged consumers to stop using any of the recalled products.
“Make sure that these recalled products are not still present, aren’t hiding on a shelf,” Spalding Walters said. “Anytime a product is recalled, there’s always a chance that it’s going to be still in homes and be used months or years down the road.”
Symptoms of an eye infection include:
- Yellow, green or clear discharge from the eye.
- Eye pain or discomfort.
- Redness of the eye or eyelid.
- Feeling of something in your eye (foreign body sensation).
- Increased sensitivity to light.
- Blurry vision.
Follow NBC HEALTH on Twitter & Facebook.
Berkeley Lovelace Jr. contributed.
Source: | This article originally belongs to Nbcnews.com