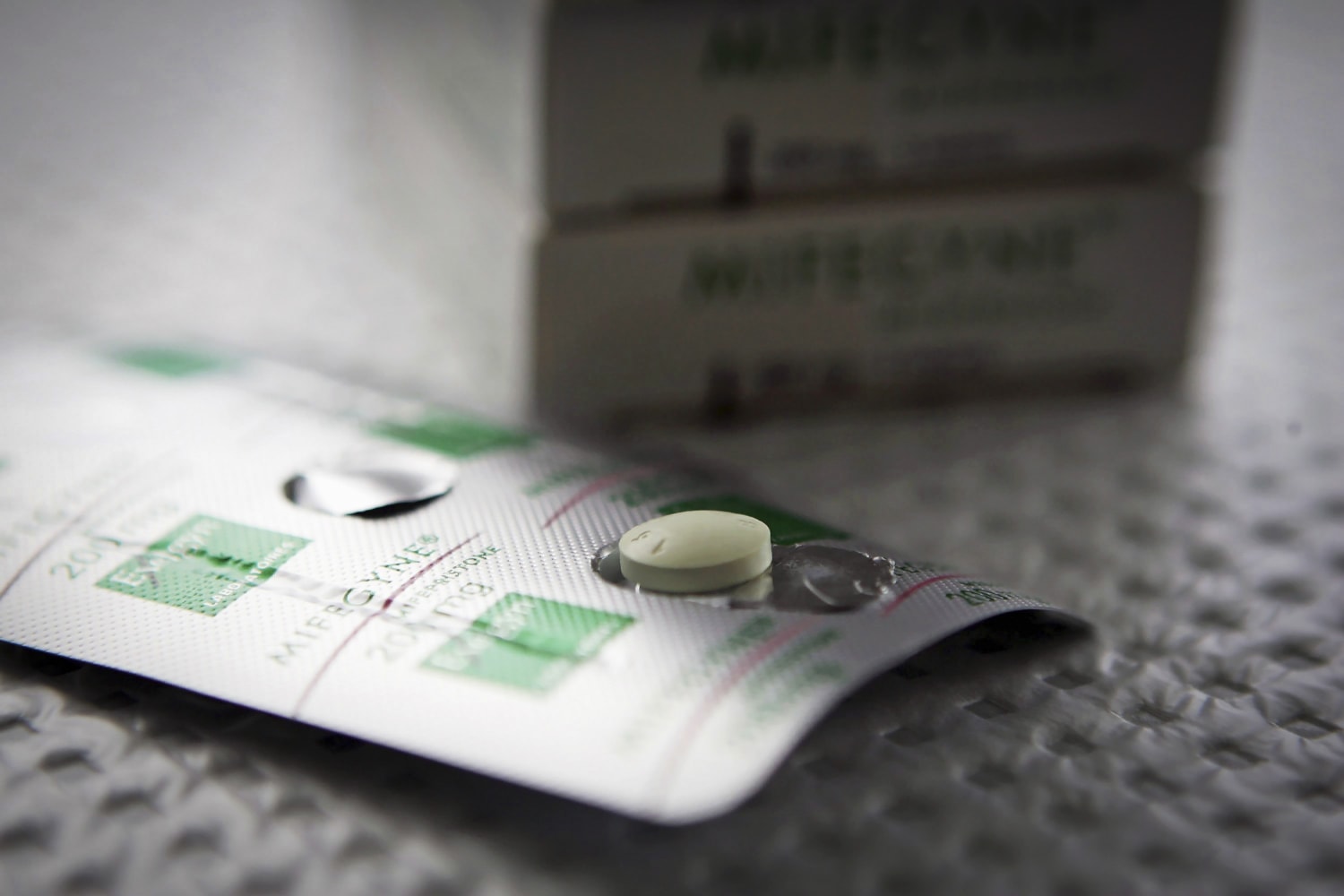
In an unprecedented move, U.S. District Judge Matthew Kacsmaryk on Friday suspended the Food and Drug Administration’s longtime approval of key abortion pill mifepristone, but gave the government a week to appeal his decision that could throw access to medication abortions into question nationwide.
The FDA approved mifepristone more than 20 years ago to be used in combination with a second drug, misoprostol, to terminate pregnancies at up to 10 weeks. Over half of U.S. abortions are done by medication abortion, according to the Guttmacher Institute, a research group that supports abortion rights. The pills have become increasingly significant in the fights over abortion access that have ensued since Roe v. Wade was overturned.
A coalition of anti-abortion groups, collectively called the Alliance for Hippocratic Medicine, sued in November challenging the process through which the FDA evaluated and approved mifepristone. They argue that the government did not adequately assess the drug’s safety and should not have made it accessible via telehealth during the pandemic.
The plaintiffs sought an injunction to halt the use of mifepristone nationwide while the case plays out.
The judge on Friday halted the original approval of mifepristone, but he has also decided to keep his ruling on hold for seven days to give the government time to appeal.
“The Court does not second-guess FDA’s decision-making lightly,” Kacsmaryk wrote. “But here, FDA acquiesced on its legitimate safety concerns — in violation of its statutory duty — based on plainly unsound reasoning and studies that did not support its conclusions.”
Without an appeal, the ruling could ultimately mean the standard FDA-approved regimen for medication abortion may no longer be available anywhere in the U.S. That would leave a surgical procedure or off-label use of misoprostol on its own as the only options in states where abortion is legal.
The Supreme Court is likely to have the final word on whether the Texas ruling goes into effect, with the Justice Department likely to immediately seek to halt it from going into effect. Even if the intermediate 5th U.S. Circuit Court of Appeals in New Orleans does block the ruling, then the challengers could ask the Supreme Court to overturn that decision within days.
Merle Hoffman, founder and CEO of CHOICES Women’s Medical Center in Queens, called the rollback of access to medication abortion “the most egregious intervention and engagement in medical care.”
It’s a reminder that even state protections aren’t enough to guarantee abortion access, she added.
“As far as I’m concerned, there’s no safe place anymore for women and girls in this country,” Hoffman said. “Maybe this will wake people up.”
In the lawsuit, the Alliance for Hippocratic Medicine alleges that medication abortion has “potentially serious and life-threatening effects,” although studies suggest that when mifepristone and misoprostol are used together in consultation with doctors, the regimen successfully terminates pregnancies up to 99.6% of the time and has a 0.4% risk of major complications.
In court filings, the FDA said that it “extensively reviewed the scientific evidence and determined that the benefits of mifepristone outweigh any risks” and that the “public interest would be dramatically harmed” if the drug were removed from the market.
It also called the lawsuit “extraordinary and unprecedented.”
There is little legal precedent for a court to overturn an FDA approval of a drug.
Minutes after the Texas ruling, in a separate case, a federal judge in Washington state issued a preliminary injunction, barring the FDA from “altering the status quo and rights as it relates to the availability of mifepristone.”
A coalition of Democratic attorneys general in February challenged the FDA’s new proposed regulations on mifepristone and asked the court to remove “unnecessary” restrictions that would limit access to it.
The ruling from the judge in Washington state applies only to the states that sued: Washington, Oregon, Arizona, Colorado, Connecticut, Delaware, Illinois, Michigan, Nevada, New Mexico, Rhode Island, Vermont, Hawaii, Maine, Maryland, Minnesota, Pennsylvania and Washington, D.C.
Earlier in his career, Kacsmaryk, a Trump appointee, represented a Christian conservative legal group called First Liberty Institute. The group sued the federal government challenging the part of the Affordable Care Act that required employers to provide free insurance coverage for birth control.
Misoprostol, which is not affected by the injunction, can be used on its own to terminate pregnancies. But it is not FDA-approved for such use without mifepristone — doctors would have to prescribe it off-label. Some abortion providers said they intend to do that if access to mifepristone is cut off, even though the one-drug approach has been shown in clinical trials to be slightly less effective than the two-pill regimen.
Laura Jarrett and Aria Bendix contributed.
Source: | This article originally belongs to Nbcnews.com