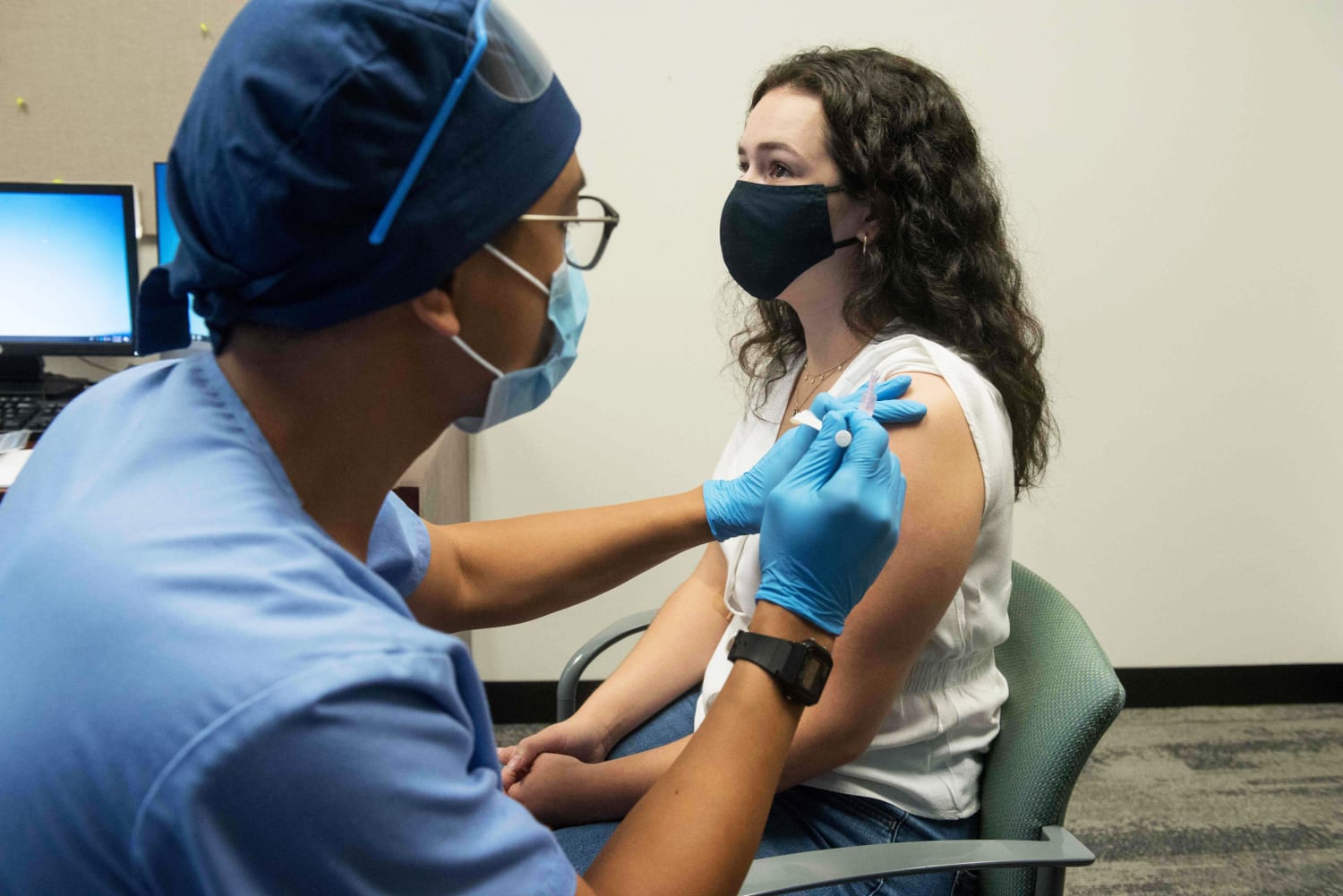
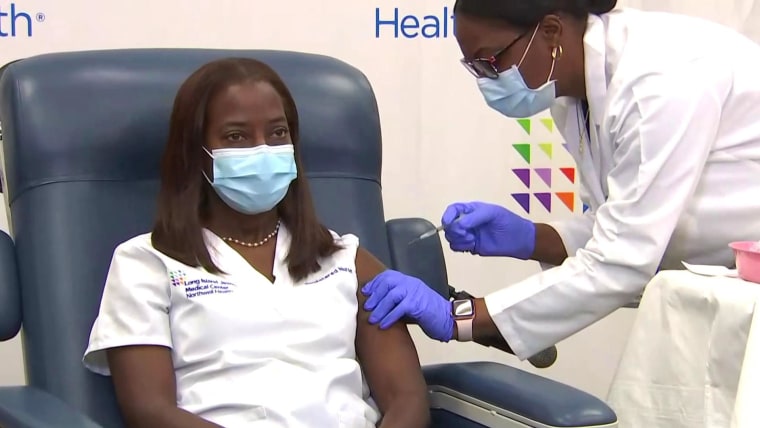
As the first Covid-19 vaccines were given in the United States on Monday, millions more doses entered the queue for nationwide distribution.
An additional 581 shipments are scheduled for delivery later this week, Army Gen. Gustave Perna, the chief operating officer of Operation Warp Speed, said during a media briefing Monday.Those deliveries will follow a previously announced 636 shipments, set to arrive by Wednesday. Each shipment contains about 1,000 doses.
Full coverage of the coronavirus outbreak
And Operation Warp Speed officials have already planned for the widespread distribution of a second vaccine, made by Moderna, though it has not yet been granted emergency use authorization by the Food and Drug Administration.
“It is a constant flow of available vaccines,” Perna said. His team is expected to update the nation on the number of available shots each Friday moving forward.
Shipments this week are expected to total 2.9 million doses of the Pfizer vaccine, which the FDA greenlighted for emergency use last Friday night. An additional 2.9 million doses are on standby for recipients to get a second dose, three weeks from now.
Phase 3 clinical trials found the vaccine is 95 percent effective across a variety of age, racial and ethnic groups when given in two doses.
Front-line health care workers are the first to receive the vaccine this week, followed by those in long-term care facilities.
Secretary of Health and Human Services Alex Azar said those shots are expected in the coming days.
“Literally by next week,” Azar said, “pharmacy chains should have the ability to execute vaccination programs daily in thousands of long-term care and assisted living facilities.”
It is highly likely that the FDA will grant emergency use authorization to drugmaker Moderna this week.
The agency is expected to release documents detailing full safety and efficacy data for Moderna’s vaccine Tuesday, ahead of a Thursday meeting of independent advisers to the FDA. The meeting of experts is anticipated to mirror last week’s meeting that scrutinized the Pfizer vaccine. The vaccines from both drugmakers use similar technology and have shown a similar level of effectiveness.
If all goes according to plan, Perna said the goal “would be for the Moderna product to be available this time next week across the United States.”
Nearly 6 million doses of the Moderna vaccines have been earmarked for distribution, Perna said, and are expected to be shipped to 3,285 locations across the country.
Hospitals are already preparing for those additional doses from Moderna.
Onisis Stefas, chief pharmacy officer for Northwell Health in New York, said the state’s health department has already informed him that his hospital system will be allocated the Moderna vaccine if and when it receives emergency use authorization.
Download the NBC News app for the latest news on the coronavirus
Two more Covid-19 vaccines are in the pipeline and are currently in pivotal Phase 3 clinical trials in the U.S. They are from AstraZeneca and Johnson & Johnson.
Preliminary results from Johnson & Johnson are expected in the first part of January, Moncef Slaoui, chief science adviser for Operation Warp Speed, said during the media briefing Monday. “If all goes according to plan,” he said, “those shots could roll out in February.”
AstraZeneca’s results could come as soon as February, with a potential March launch for distribution.
Azar predicted that with these four vaccines, at least 100 million shots of the vaccine could be administered by spring of 2021.
Source: | This article originally belongs to Nbcnews.com