Pharma giant AstraZeneca has signed a huge deal with a UK start-up to create treatments for diabetes and other autoimmune diseases.
The FTSE 100 firm will team up with London-based Quell Therapeutics to collaborate on research and the licensing of any new medicines.
The aim is to develop treatments for Type 1 diabetes and inflammatory bowel disease through the use of genetically engineered ‘regulatory T-cells’ – ‘Tregs’ for short.
Tregs help to stop the immune system from overreacting in areas of the body affected by a disease. Quell’s technology is an extension of therapies which have proven successful in treating cancer.
Astra says it will pay Quell £68m upfront to help fund the research.


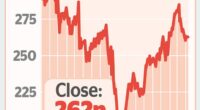
Deal: AstraZeneca will team up with London-based Quell Therapeutics to collaborate on research and the licensing of any new medicines
The start-up will also be eligible for over £1.6billion in development milestone payments and royalties if any drugs are approved for sale.
Mene Pangalos, executive vice-president of biopharmaceuticals research at Astra, said the deal would allow it to ‘explore the untapped potential with Treg cell therapies’.
Quell boss Iain McGill added: ‘This collaboration builds on our pioneering work to develop exquisitely engineered, multi-modular Treg cell therapies for immune disorders.’
Quell was founded in 2019 by six immunologists from King’s College London, University College London and Hannover Medical School.
Prior to the deal with Astra, it raised about £175m from a consortium of investors and is valued at about £239m.
A major backer is London-listed firm Syncona, a trust that invests in companies focused on cell and gene therapies. The firm owns just over a third of Quell.
‘Astra’s expertise and capability will be instrumental in driving these important programmes forward,’ said Martin Murphy, who is chairman of both Syncona and Quell.
News of the tie-up came as Astra announced that the US Food and Drug Administration (FDA) had voted to recommend its drug nirsevimab as a treatment to prevent respiratory syncytial virus (RSV) in infants.
RSV is a common infection that causes cold-like symptoms. Most people recover within two weeks but the illness can become serious in infants and older adults and may lead to lung infections and pneumonia.
If nirsevimab is approved, it will be the first medicine designed specifically to protect infants from RSV.
‘We look forward to continuing to work with the FDA to complete their expedited review, and we hope to see nirsevimab available as soon as possible given the significant burden of RSV in infants,’ said Iskra Reic, Astra’s executive vice-president of vaccines and immune therapies.